Project Management Service
We manage projects and programs for pharmaceutical and life science companies worldwide, with certified program managers and project managers with highly specialized skills.

Request more information today
Project Management Service
We manage projects and programs for pharmaceutical and life science companies worldwide, with certified program managers and project managers with highly specialized skills.
E2E PRODUCT LIFECYCLE MANAGEMENT
Specialized consulting
S4BT’s Project Management Service is a highly specialized consulting service for end-to-end project management at all stages of a product’s life cycle: research and development, commercialization, divestment or delisting.
The S4BT consulting team consists of certified program managers and project managers (PMP®, Yellow Belt, ISIPM®, PRINCE2® Foundation, CAPM®, CSCP®) with specialized technical know-how and robust experience in executing projects alongside pharmaceutical companies and CDMOs.
E2E PRODUCT LIFECYCLE MANAGEMENT
Specialized consulting
S4BT’s Project Management Service is a highly specialized consulting service for end-to-end project management at all stages of a product’s life cycle: research and development, commercialization, divestment or delisting.
The S4BT consulting team consists of certified program managers and project managers (PMP®, Yellow Belt, ISIPM®, PRINCE2® Foundation, CAPM®, CSCP®) with specialized technical know-how and robust experience in executing projects alongside pharmaceutical companies and CDMOs.
R&D
New Product Introduction (NPI)
Value Chain Management (VCM)
Delisting & Divesting
Tech Transfer*
Product Quality Management (PQM)
the service in detail
E2E Product Lifecycle Management
Our activities include:
- Project Start up
- Planning
- Execution
- Monitoring
- Close out
* For Technology Transfer, we offer consulting services either as a Project Manager or Technical Lead.
- Documentation Production
- Project Charter
- Risk Management
- Stakeholder engagement
- KPI Monitoring
- Cross Multifunctional Team Management
- Communication Management
R&D
New Product Introduction (NPI)
Value Chain Management (VCM)
Delisting & Divesting
Tech Transfer*
Product Quality Management (PQM)
the service in detail
E2E Product Lifecycle Management
Our activities include:
- Project Start up
- Planning
- Execution
- Monitoring
- Close out
* For Technology Transfer, we offer consulting services either as a Project Manager or Technical Lead.
- Documentation Production
- Project Charter
- Risk Management
- Stakeholder engagement
- KPI Monitoring
- Cross Multifunctional Team Management
- Communication Management
Why Choose Project Management Service
Experience in the field
We have been operating for about 30 years in the pharmaceutical and life science sectors.
Our consultants are progtam and senior project managers with robust experience managing projects in national and international settings for large companies in the industry.
Flexibility
We move with flexibility of time and place to meet customers' needs.
We routinely support international travel to reach plants.
We also have technology to work both remotely and from the customer's site without any difficulty.
Targeted Investment
With The PM Service, the client decides on the job profile and how long he or she will need it, resulting in: a reduction in fixed Personnel costs; flexible production capacity; and tailored specific skills.
Specialized Technical Know-How
S4BT consultants have excellent knowledge of cGXP, manufacturing processes, Technology Transfer, and change management.
We also invest in the continuous training of our consultants, who have highly specialized know-how in Pharma & Life Science and are always up-to-date with the current environment.
CONTINOUS IMPROVEMENT
Relying on the expertise and know-how of S4BT Project Management Service consultants not only helps complete a Project but also provides advice for continuous improvement of flows and processes.
Service Excellence
With the Project Management Service, client companies have a Business Unit Manager who follows the consultant's path and is a constant point of contact to meet the client's needs, ensuring a personalized customer experience and high performance.
Experience in the field
We have been operating for about 30 years in the pharmaceutical and life science sectors.
Our consultants are progtam and senior project managers with robust experience managing projects in national and international settings for large companies in the industry.
Flexibility
We move with flexibility of time and place to meet customers' needs.
We routinely support international travel to reach plants.
We also have technology to work both remotely and from the customer's site without any difficulty.
Targeted Investment
With The PM Service, the client decides on the job profile and how long he or she will need it, resulting in: a reduction in fixed Personnel costs; flexible production capacity; tailored specific skills.
Specialized Technical Know-How
S4BT consultants have excellent knowledge of cGXP, manufacturing processes, Technology Transfer, and change management.
We also invest in the continuing education of our consultants, who have highly specialized know-how in Pharma & Life Science and are always up-to-date with the current environment.
CONTINOUS IMPROVEMENT
Relying on the expertise and know-how of S4BT Project Management Service consultants not only helps complete a Project but also provides advice for continuous improvement of flows and processes.
Service Excellence
With the Project Management Service, client companies have a Business Unit Manager who follows the consultant's path and is a constant point of contact to meet the client's needs, ensuring a personalized customer experience and high performance.
Our experience in the field
until
0 mlnuntil
0 mlnTechnology Transfer of the Manufacturing Process of an Oral Solid Product
Client:
Multinational pharmaceutical company that has always been engaged in research, development and commercialization of innovative drugs with one of the most modern and state-of-the-art factories.
For the Group, the transfer of products from one country to another plays a strategic role that requires expert management of the task.







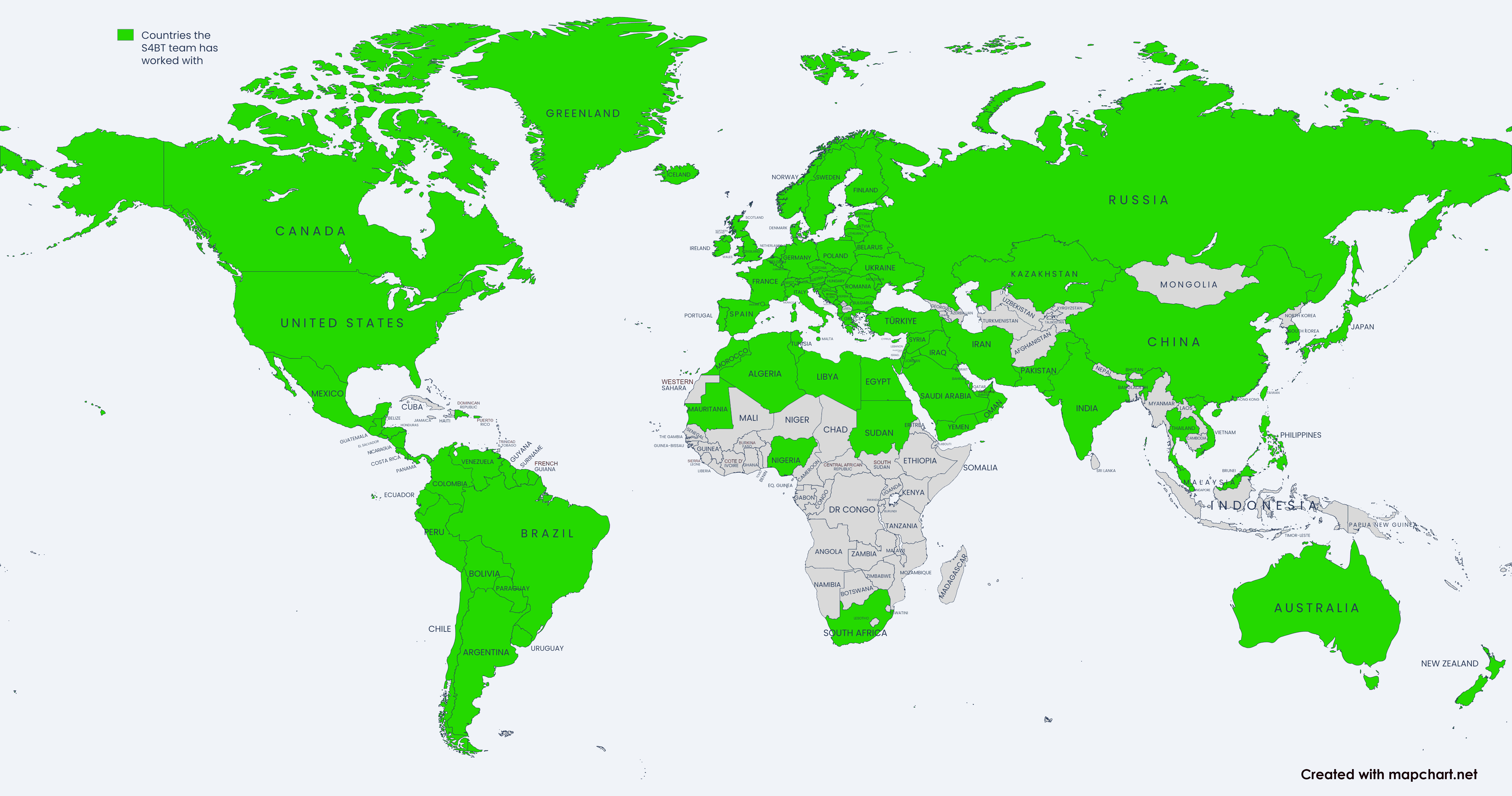
We follow projects around the world
About Us
S4BT specialists make essential contributions in all Technology Transfer projects in which they are involved. I am extremely satisfied with the technical expertise and management skills demonstrated





We are a company of more than 80 specialists and have been a reliable partner of excellence for Pharma & Life Science companies for about 30 years, to whom we provide software solutions and specialized consulting.
S4BT Consulting has specialized know-how in the areas: Quality Assurance; Project Management; Technology Transfer; Process, Cleaning & Packaging Validation; NPI; Packaging Engineering; Equipment & Facilities Qualification; CSV & Data Integrity.
PRAGMA4U is the platform for digitizing processes in regulatory compliance.
eQMS, DMS, LMS, WFMS multiple technologies in one platform.
PRAGMA4U is flexible, adapts to the needs of companies of any size, and enables sustainable growth.
Designed with human centerd approach, it offers a path to process optimization.